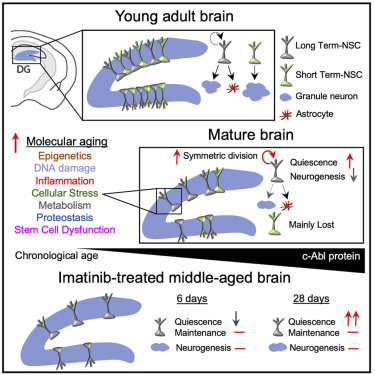

Aging is the progressive loss of physiological function due to accumulating cellular damage (Kennedy et al., 2014; López-Otín et al., 2013). This process occurs at a gradual and asynchronous rate where specific cells and then organ systems lose homeostasis (Almanzar et al., 2020; Negredo et al., 2020; Schaum et al., 2020). Aging has been historically investigated in chronological terms, with the majority of studies comparing young and old organisms. Yet, approaches to prevent aging at younger ages have recently become more common (Belsky et al., 2015; Gillman, 2005). However, progress in slowing or reversing a decline in tissue function has been hampered by an inability to determine when and which cells begin to exhibit cellular aging (López-Otín et al., 2013; Solanas et al., 2017).

Adult neurogenesis persists throughout life in the subgranular zone in the dentate gyrus of the hippocampus and is highly compromised during chronological aging (Kirschen et al., 2019; Kuhn et al., 2018; Toda and Gage, 2018).
Aged animals have significantly lower neural stem cell (NSC) numbers and less stem cell proliferation, neuronal differentiation, and newborn neuron survival compared to younger animals (Encinas et al., 2011; Heine et al., 2004; Kuhn et al., 1996; Ziebell et al., 2018). Further, NSCs in old animals exhibit hallmarks of molecular aging, including alterations to proteostasis and inflammation (Kalamakis et al., 2019; Leeman et al., 2018; Negredo et al., 2020). Yet, the hippocampus experiences a loss of neurogenesis early in the mature brain of rodents (Ben Abdallah et al., 2010; Morgenstern et al., 2008) and by middle age in humans (Knoth et al., 2010; Moreno-Jiménez et al., 2019; Spalding et al., 2013). This decline is accompanied by epigenetic loss of DNA demethylation (Gontier et al., 2018), suggesting NSCs could molecularly age at early stages of chronological aging.
However, cellular origins driving the early neurogenesis decline remain unclear. In one scenario, NSCs have been found to drop in number in the young hippocampus (Gontier et al., 2018; Lugert et al., 2012). This is supported by the presence of a finite NSC pool containing limited self-renewal capability (Encinas et al., 2011; Pilz et al., 2018). Consistently, forced accelerated neurogenesis (Ehm et al., 2010; Jones et al., 2015; Renault et al., 2009) and pathological conditions (Mu and Gage, 2011; Sierra et al., 2015) can result in premature NSC depletion. In another scenario, studies (Bonaguidi et al., 2011; Dranovsky et al., 2011; Licht et al., 2016) indicate that self-renewing NSCs are maintained for prolonged periods and neurogenesis could instead decline during aging due to an increase in NSC quiescence (Hattiangady and Shetty, 2008; Ziebell et al., 2018). While NSCs as a population clearly undergo early age-related changes, when and how specific NSC subpopulations begin to exhibit dysfunction are unclear. In this study, we used single-cell approaches to investigate the cellular and molecular mechanisms that initially compromise adult neurogenesis. We determined that NSC subpopulations undergo asynchronous decline and exhibit early molecular aging. We further show that targeting these cellular aging mechanisms in the middle-aged brain can partially overcome age-related NSC dysfunction.